Today’s most advanced technology, from crafting tiny computer chips to engineering super-strong materials, hinges on understanding how electrons interact with molecules. But there’s a problem: when molecules contain “heavy” atoms (anything heavier than Argon), calculations become incredibly difficult and slow, owing to the sheer number of electrons present.
Heavy atoms have so many electrons that:
- They move fast: Core electrons in heavy atoms move so quickly that we need special, complex math to describe them accurately (relativistic effects).
- Calculations may be slow: More electrons mean much bigger, slower computer calculations, often requiring excessive resources. This adds a barrier to discovery by making simulations more costly and time-consuming.
Our Solution: ECPs in QEC 2.0
At Quantemol, we’re all about speeding up science and technology. That’s why we’re excited about a big new improvement in our Quantemol Electron Collisions (QEC) software: we’ve added Effective Core Potentials (ECPs).
QEC is our specialised software that calculates how electrons “bounce off” molecules. With ECPs, we’ve found a clever way to make these calculations much easier.
Think of ECPs as a “smart shortcut.” Instead of making the computer track every single electron in a heavy atom, ECPs handle the inner, chemically inert electrons in a simplified way. This lets QEC focus processing power on the outer, chemically active electrons.
The result? We drastically cut down computational needs, while still obtaining accurate results! For example, with a molecule like CH3Br: using ECPs on the heavy Bromine atom reduces the number of electrons that the computer has to handle from 44 down to just 16. That’s a huge saving, making these tough calculations possible on everyday computers. In addition, fast-moving core electrons are treated with a relativistic correction factor, improving the quality of the physical model.
How to use ECPs in QEC
ECPs are easy to activate in QEC. We’ve designed QEC so that users can simply select atoms to which they want to apply ECPs from a dropdown menu, and that’s it! Simply click on ‘Advanced Options’ on our Molpro Setup page, and select ‘Use an ECP’. This provides a list of all atoms (except hydrogen and helium), so that the user can select where ECPs should be applied. Figure 1 shows how this appears in the case of Br2.
Figure 1: Selection of an ECP for each Br atom in the Molpro Setup Advanced Options.
Case Study: Br2 – Proving It Works
To make sure our new ECP feature was accurate, we needed a solid test. We picked a challenging molecule, Br2. Why Br2? Because it’s a “heavy” molecule with lots of inner electrons, that can be simplified using ECPs; but, it’s also small enough that we could run calculations the ‘old way and the new way. This allowed us to directly compare the results.
The comparison showed great news!
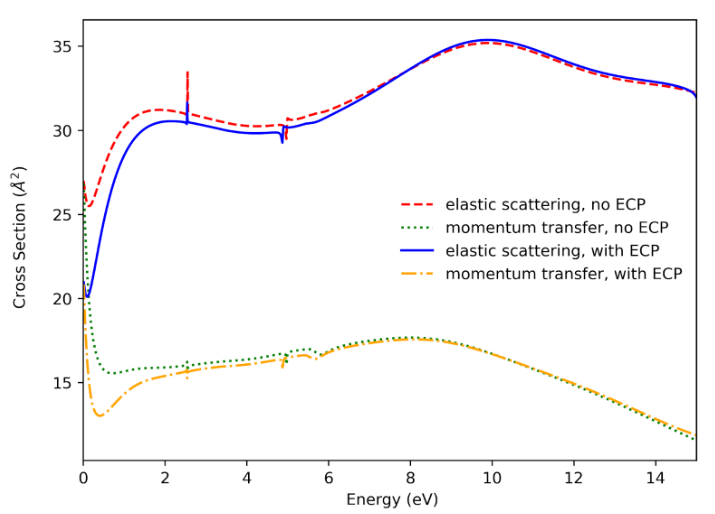
Figure 2: Results for Br2 showing how electrons interact with the molecule.
Our new QEC software using ECPs (blue and yellow lines) gave results that are in good agreement with our established, trusted method (red and green lines). This tells us the new software is accurate and reliable.
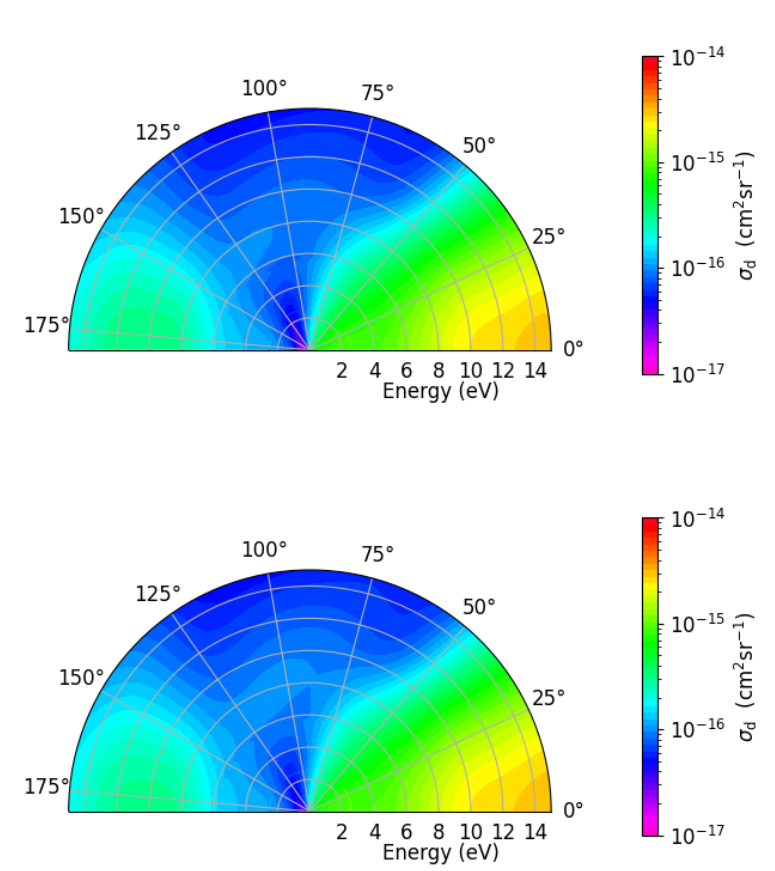
Figure 3: Results for Br2 showing the energies and angle of emission (relative to the internuclear axis) of scattered electrons calculated with ECPs (top) and without ECPs (bottom).
Figure 3 confirms this accuracy for even more specific details of how electrons interact with the molecule, i.e, the scattering angles with and without ECPs. It means our ECPs are capturing the important scattering behaviors.
An additional benefit in this case was the speedup achieved by using ECPs. The calculation without ECPs took around 20 minutes on a 28-core workstation, while the calculation using ECPs needed only 6 minutes. What used to be a huge computational challenge is now a practical and efficient simulation.
The addition of ECPs to Quantemol QEC opens up new possibilities for scientists and engineers in fields like semiconductor manufacturing and advanced materials. It allows them to simulate how electrons interact with molecules that contain heavy elements – challenges that were once too complex or costly to tackle.
Ready to make your research faster and smarter, especially when dealing with heavy elements? Visit quantemol.com to learn more, or contact us for a quick chat about your needs. And don’t forget to follow Quantemol on LinkedIn for more exciting updates!
By Greg Armstrong & Annie Laver

Dr Greg Armstrong
PRINCIPAL COMPUTATIONAL MOLECULAR PHYSICIST

Annie Laver
SCIENTIFIC COMMUNICATIONS ADMINISTRATOR
